A pre-term baby’s chances to survive and thrive depend on being able to absorb nutrients, so that they can grow—and grow stronger. For the first weeks of life, adequate nutrition is critical for brain and vital organ development. Preterm babies are usually born before their digestive systems are fully developed. Too young to be able to coordinate sucking, swallowing, and breathing, they must be fed by a tube, which enters directly to their gut via the nose or mouth. But even once food reaches their digestive tract, a condition called intestinal malabsorption limits how much nutrition they can properly absorb.
The current standard of care is to place infants on central line nutrition (also called parenteral nutrition or PN) which "bypasses" their immature intestines and delivers nutrients directly to the bloodstream, until their digestive systems are capable of absorbing sufficient nutrition. However, central line nutrition poses high risks of significant complications, mainly infections including sepsis, PN associated liver disease and growth failure.
On the other hand, overloading the infants' GI tract before it is mature enough can result in the most feared complication of prematurity-necrotizing enterocolitis (NEC). This condition is defined as an ulcerative inflammation of all layers of the intestinal wall. The exact pathophysiology of NEC is not yet fully understood. Early signs are not specific and range from localized intestinal symptoms to generalized signs of sepsis. This is a life-threatening complication with mortality rates that reach as high as 42% in the smallest babies¹.
The younger the baby and the more severe its intestinal malabsorption is, the longer they tend to be fed via PN. However, prolonged PN is associated with significant complications, including clots, atrophy of the intestines, liver disease and life-threatening infections like sepsis. Babies of extremely low birth weight are at the highest risk for developing one or more PN associated complications, while it is still the only way to feed them. As a result- there is a high unmet need for a method that makes the GI maturation process more efficient so that infants can wean-off of PN more quickly, and the baby can receive all its nutrition via GI tract.
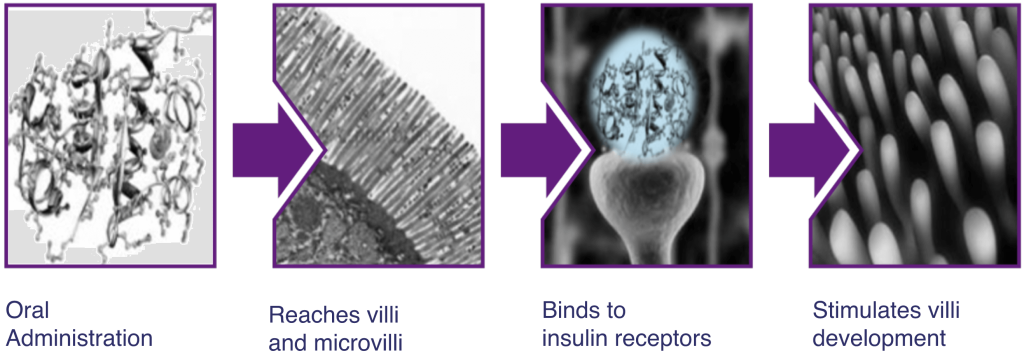
This is where ELGAN comes in. Our lead product (ELGN-GI) is an oral formulation of insulin, acting locally in the gut. It is a highly soluble powder that can be added to the baby's nutrition, both breast milk and formulas. The treatment goal with this product is a faster and more efficient development of the intestines of these premature babies.
Physiologically, insulin is found in amniotic fluid and in breast milk in the first few days of life. Accumulating evidence across several mammalian species indicates that insulin has important physiological effects on intestinal growth, cell maturation, and enzyme expression. Immediately after birth, a newborn infant’s GI tract is exposed to insulin via colostrum (breast milk in the first days of life). Importantly, in utero the GI tract is also exposed to the insulin present in amniotic fluid and consumed by the fetus, resulting in a constant influx of insulin stimulating the GI tract.
ELGN-GI in fact mimics the natural exposure to insulin that would have occurred in case of a full-term pregnancy. The product is given orally, and its effect is local and limited to the GI tract, with no systemic absorption (does not enter the blood stream).
As a result of faster and more efficient development of their GI tract, babies treated with ELGN-GI reach their nutrition goals sooner and their time on PN is shorter. The product was tested in several clinical studies, including a successful phase III², and is currently starting a second registrational phase III study.
ELGN-GI is potentially beneficial for another condition- Short Bowel Syndrome (SBS), which most commonly occurs following extensive small bowel resection. SBS is the most common cause of intestinal failure in children and a very complex disorder that affects normal intestinal physiology. This results in nutritional, metabolic, and infectious consequences, like excessive fluid loss, nutrient malabsorption, electrolyte abnormalities, increased susceptibility to infections, parenteral nutrition associated complications and affects weight gain and growth.
In children, SBS is debilitating and uniformly fatal without treatment.
The primary goal of treatment is to restore enteral autonomy and reduce long-term dependence on parenteral support by increasing the absorptive potential of the remnant intestine. Few conditions in pediatric gastroenterology pose as great a challenge as short bowel syndrome, with high morbidity and mortality despite available advanced treatment modalities.
ELGN- GI can potentially increase the ability of residual bowel to adapt, thus reducing parenteral nutrition (PN) and central line dependence, leading to less complications and better motor, social and mental development. A pilot study already tested oral insulin for pediatric SBS and the results suggest that oral insulin administration in children with SBS may provide meaningful clinical benefit to and is not associated with short-term side effects³.
ELGN-GI is an enterally (orally or via tube) administered insulin, acting as a targeted local GI therapy to accelerate intestinal development. It is an insulin formulation with excipients which are approved for infant consumption. The product is highly soluble and can be reconstituted in all types of infants' nutrition (human milk and formula) or in water.
When ELGN-GI reaches the GI, the insulin can bind to specific receptors in the bowel and stimulate its development and maturation, as it would have occurred in case of a full-term pregnancy. A more rapid gut maturation enables premature infants to reach full enteral feeding faster, meaning that they can consume and absorb all the needed nutrition via their GI tract.
ELGN-GI dose is similar to the physiological levels of insulin found in colostrum (human milk produced in the first few days after delivery). Its effect is local and limited to the infant's intestines, with no systemic exposure (the product isn't absorbed from the baby's gut into the bloodstream).
ELGN-GI is simple to prepare and administer in the NICU, together with the infants nutrition.