Retinopathy of prematurity (ROP) is a major cause of severe visual impairment or blindness in infants born prematurely, with approximately 50,000 infants becoming blind worldwide each year¹. The disease is characterized by abnormal vascular proliferation in the immature retina. Extreme prematurity (<28 weeks gestational age, <1250 gr birth weight), growth restriction, male sex, hyperoxia, and septicemia are most consistently associated with the development of ROP. Although changes in clinical practice, namely more judicious oxygen administration, have resulted in a decreased incidence of ROP in developed countries over the past several years, affected infants are still at risk for subsequent ophthalmologic complications. This condition remains the third leading cause of childhood blindness.
In utero, angiogenesis begins at 16-17 weeks gestational age, with new vessels budding from existing vessels. Several vasoactive factors, including insulin like growth factors and erythropoietin, stimulate new vessels formation and by 40 weeks gestational age they are fully developed.
In premature infants, especially ones born earlier than 28 weeks, the retinal blood vessels are still immature and vulnerable to oxidative damage.
ROP has two phases: transition from in utero environment results in loss of placental and maternal growth factors and in parallel- exposure to higher oxygen levels, leading to arrest and retraction of the developing retinal vessels. As the infant matures, the avascular retina becomes metabolically active, leading to the second phase of ROP - hypoxia, upregulation of vascular growth factors and an abnormal overgrowth of retinal blood vessels.
ELGN-EYE is a unique formulation based on insulin that contains the same compounds present in the amniotic fluid during pregnancy. The therapeutic goal here is to promote normal growth and development of retinal blood vessels, reduce oxidative stress and toxicity and reduce inflammation. The insulin in ELGN-EYE stimulates blood vessels and retinal layers to continue to develop via an insulin receptor mediated effect. Insulin also has anti-inflammatory properties² and it reduces reactive oxygen species (ROS), thus reducing oxidative stress and damage³.
ELGN-EYE is an insulin-based eye-drops formula with supportive endogenous excipients at physiological levels.
The drug is intended for topical administration and is designed as a nano-emulsion, which is a unique platform for delivering insulin to the interior eye compartments.
Insulin plays a key role in retinal development and angiogenesis. Insulin receptors are expressed on both the vasculature and neurons of the retina. In the ocular and neural tissues Insulin plays a DNA synthesis role rather than metabolic management role, as it does in skeletal muscles.
ELGAN-EYE is administered, allowing a receptor-mediated effect and promoting vascular growth and cells viability. The local administration provides higher levels of exposure specifically in the target organ, leading to better efficacy. The novelty of the therapy is its enhanced drug penetration profile using a nano-emulsion formulation with excipients that "pull" the drug to the posterior side of the eye.
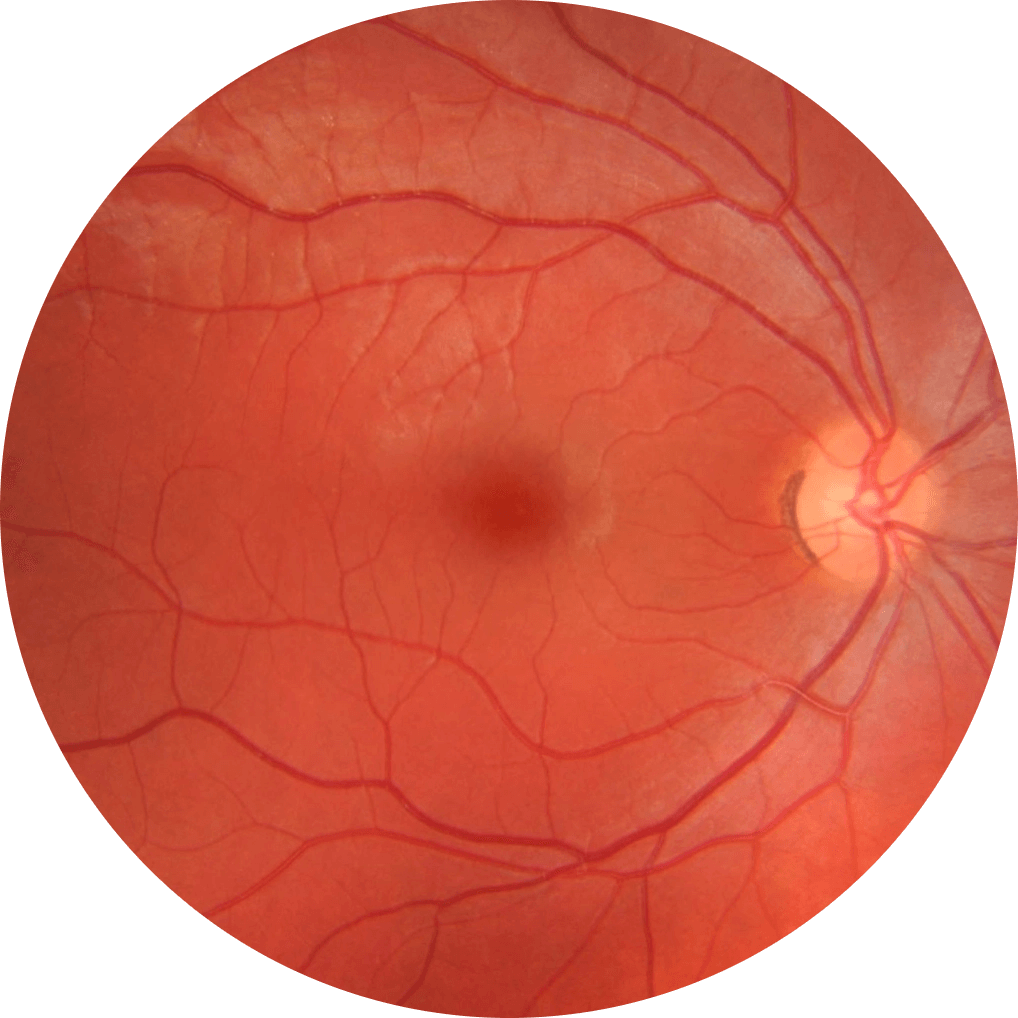
Developed
Retina
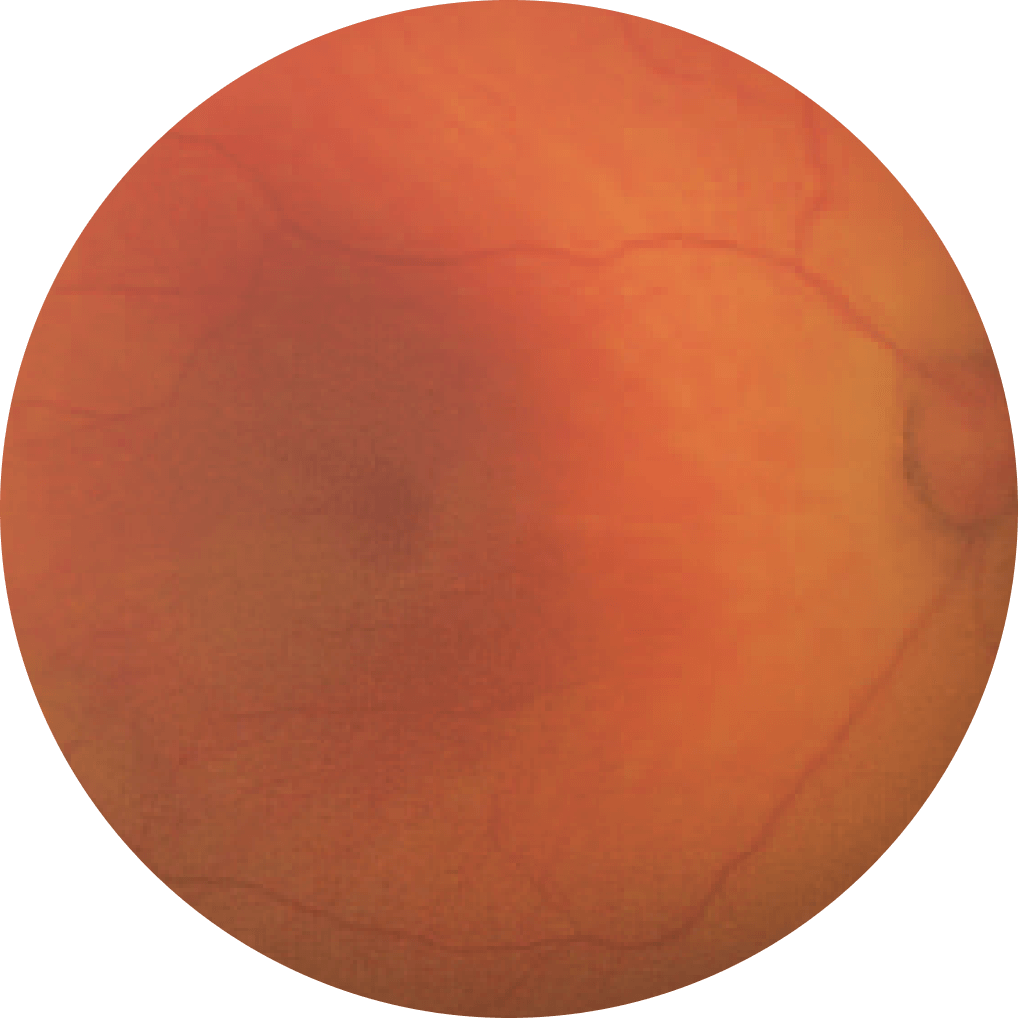
Premature
Retina

Local Nano-Emulsion therapy for prevention
of vision impairment:
